Study of motion or in other words Dynamics is investigating why things move and what is the behavior behind that. The prefix thermo indicates heat, so thermodynamics is the investigation of what urges warmth to move in the way that it does.
Laws of Thermodynamics let us know about the flow of heat. The laws of thermodynamics are somewhat odd however very important when it comes to physics homework help. They are four of them. Let us check out them individually.
Zeroth Law
It states that “If system A is at thermal equilibrium with system B, and B is at thermal equilibrium with system C, then A is at thermal equilibrium with C.” This dives more into logic than physics. In other words, if A & B have the same temperature, and B and C have the same temperature, then A and C have the same temperature as well.
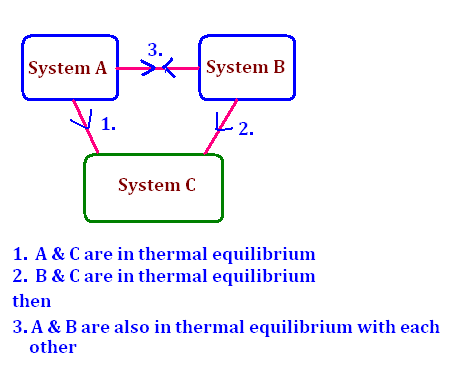 This explains it that heat transfer taking place from a hot object to a cold object when placed together, until they reach a state of equilibrium. Get yourself an online physics tutor to help you out with the subject.
This explains it that heat transfer taking place from a hot object to a cold object when placed together, until they reach a state of equilibrium. Get yourself an online physics tutor to help you out with the subject.
#Quantum #Physics And Major Findings In The Field http://t.co/06yRuTu8WQ #physicshelp #BackToSchool pic.twitter.com/jqoK3wp4BK
— Tutor Pace (@TutorPace) September 1, 2015
First Law Of Thermodynamics
Consider a secluded framework where energy and heat neither leave nor enter the framework. Such a framework is doing no work, yet we take up with it a certain amount of internal energy, U, which is identified with the kinetic energy of the atoms in the framework, and in this manner to the framework’s temperature. This internal energy is referred similar to potential energy due to the property of a framework that is doing no work, however can possibly do work.
The first law lets us know that the potential energy of a framework builds if warmth is added to the framework or if work is done on the framework and reduces if the framework emits warm or does work. We can express this law as a mathematical statement where U connotes internal energy, Q implies heat, and W means work.  The First Law is simply one more method for expressing the law of conservation of energy. Both work and heat are types of energy forms, so any warmth or work that goes into or out of a framework must influence the potential energy of that framework.
The First Law is simply one more method for expressing the law of conservation of energy. Both work and heat are types of energy forms, so any warmth or work that goes into or out of a framework must influence the potential energy of that framework.
Learn Physics The Easiest Way with Online Physics Tutor http://t.co/PXL3NWpRRK #backtoschool #physicshelp
— Tutor Pace (@TutorPace) September 1, 2015
Second Law Of Thermodynamics
The second law is most well known for its entropy and formulation. The word entropy was instituted in the nineteenth century as a specialized term for discussing issue.
Pouring a tablespoon of salt and after that a tablespoon of pepper into a jug. At to start with, there will be two different stacks: salt as well as pepper. When you shake up the blend, the grains of salt and pepper will combine. No measure of shaking will then help you isolate the blend of grains again into two separate varieties. The two different salt and pepper constitute a more organized framework than the blend of the two.
 Next, assume you drop the container on the floor. The glass will break resulting in grains of salt and pepper dispersing all over the floor. You can hold up persistently, however you’ll see that, while the glass could smash and the grains could scramble, no activity as basic as dropping a container will recover the glass to intervene again or the salt and pepper to accumulate themselves up. Your arrangement of salt and pepper in the container is more organized than the arrangement of smashed glass and dispersed condiments.
Next, assume you drop the container on the floor. The glass will break resulting in grains of salt and pepper dispersing all over the floor. You can hold up persistently, however you’ll see that, while the glass could smash and the grains could scramble, no activity as basic as dropping a container will recover the glass to intervene again or the salt and pepper to accumulate themselves up. Your arrangement of salt and pepper in the container is more organized than the arrangement of smashed glass and dispersed condiments.
Reinvent Your Physics Skills With Online #Physics Tutor http://t.co/AsA4JU9Zv9 #backtoschool #physicshelp
— Tutor Pace (@TutorPace) September 1, 2015
Third Law Of Thermodynamics
The third law of thermodynamics expresses that “the entropy of a framework at absolute zero is a well defined constant.” This is on account of a framework at zero temperature with an existence in ground state, so that its entropy is resolved just by the decline of the ground state. It is difficult to cool a substance to absolute zero.
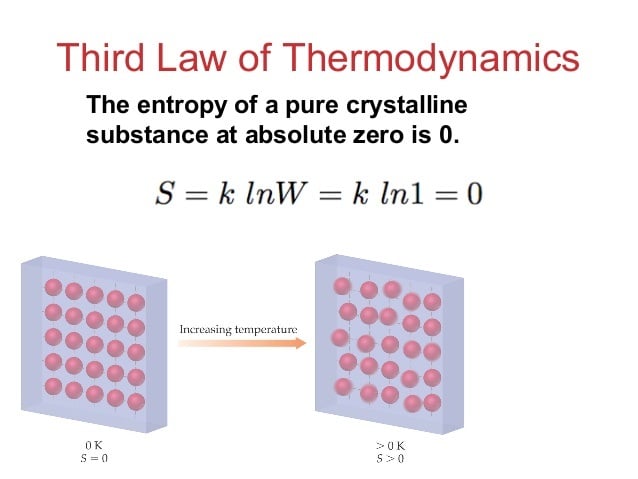 To know more on Laws Of Thermodynamics chat with live physics tutor online and get your physics assignment help from expert online physics tutors.
To know more on Laws Of Thermodynamics chat with live physics tutor online and get your physics assignment help from expert online physics tutors.

