There are certain concepts in chemistry which a student should know and they can be said as fundamental concepts. In online tutoring these concepts are stressed more so that students can understand the question easily. We all know that atoms are joined with each other with chemical bonds. Atoms are composed of electrons which revolve around the nucleus of an atom. There are two types of bonds; these are covalent bonds and ionic bonds. Whenever there is chemical reaction we know that products are formed with the help of reactants. To understand them easily we have chemical symbols and we show them with formulas to form equations.
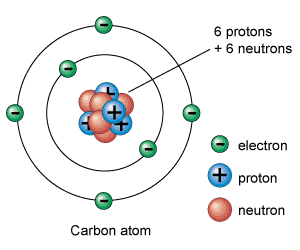 We all know that every substance is made from atoms. Atom has a nucleus in the middle and is surrounded with small particles called electrons. Electrons are negatively charged particles. In Nucleus there are protons and neutrons present. Nucleus is positively charged because protons are positively charged particles. Neutrons have no charge
We all know that every substance is made from atoms. Atom has a nucleus in the middle and is surrounded with small particles called electrons. Electrons are negatively charged particles. In Nucleus there are protons and neutrons present. Nucleus is positively charged because protons are positively charged particles. Neutrons have no charge
Now let’s know more about elements. An element has only one type of atom for example if we have an iron rod it will have only iron atoms or we can write Fe atoms. Similarly if we have carbon particles it will have only carbon atoms. Also to represent an element we use chemical symbols it can contain one to two letters but sometimes for new discovered element we have three letters. First letter of element is always in an upper case and other letters are in lower case. For example we have magnesium its symbol is written as Mg.
